
Introduction :Since the introduction of tyrosine kinase inhibitors (TKIs) the prognosis of Philadelphia positive (Ph+) diseases like chronic myeloid leukemia (CML) dramatically improved (Sasaki K, Lancet Haematol 2015). Nonetheless, myeloid blastic crisis of CML (MBC-CML) and Ph+ acute myeloid leukemia (AML) represent a clinical challenge due to their rarity, the absence of a standard therapy and mutations.
Methods :We collected clinical data of patients treated in Paris in 5 different hospitals with confirmed diagnosis in patients with CML successively transformed in MBC-CML, and in patients with MBC-CML straight away or Ph+ AML. We performed descriptive analysis as well as analysis on overall survival (OS) and event-free survival (EFS).
Results :We found 64 patients (24 F/40 M) treated since 2001 to present days. 35/64 patients had an history of CML before MBC of which 30 were diagnosed in chronic phase (Sokal risk: low 3 pts, intermediate 8 pts, high 4 pts, NA 15 pts) and 5 in accelerated phase according to ELN classification.
Lines of treatment before MBC for these 35 patients were principally TKIs, IFN, hydroxyurea and homoarringtonine: 11 pts experienced only 1 line of treatment, 15 pts 2 lines, 5 pts 3 lines, 1 pt 4 lines and 3 pts 5 lines. Best response before MBC was complete hematological remission (CHR) for 14 pts, complete cytogenetic remission (CCyR) for 7 pts, molecular remission for 6 pts (4 pts MR3, 1 pt MR4, 1 pt CMR TKI free), whereas 8 pts never gained any response.
21/64 pts were in MBC at diagnosis and 8/64 pts were classified as Ph+ AML.
At diagnosis of MBC/AML median age was 49 years old (11-73).
12/64 pts had an extramedullary/central nervous system (CNS) involvement of which 2 pts presented a solitary CNS disease and 3 patients had lesions compatible with myeloid sarcomas without marrow blasts.
46/64 pts (72%) received a classical induction therapy (mostly cytarabine + anthracycline combination) combined in 40 pts with a TKI (15 pts Imatinib, 13 pts Dasatinib, 2 Nilotinib and 10 Ponatinib). 11/64 pts (17%) started Azacytidine (1 pt in combination with Imatinib, 2 pts with Nilotinib, 6 pts with Dasatinib, 1 pt with Ponatinib and 1 pt with Venetoclax). 2/64 pts (3%) received Dasatinib monotherapy. 1/64 pt (2%) received ATRA+ATO+Dasatinib combination. 4/64 pts (6%) received only best supportive care.
At first revaluation after therapy 47 pts (78%) were in CR/CRi, 9 pts (15%) were refractory, 2 pts (3%) died during induction, 1 pt (2%) induction therapy is ongoing, in 1 pt (2%) lost to follow up.
Most patients who obtained CR/CRi after induction continued with consolidations, mostly high-dose Cytarabine associated with a TKI.
41 pts (68%) were transplanted (40 alloHSCT, 1 autoHSCT), 38 pts in first CR (CR1), 1 pt in second CR (CR2) and 2 pts in progressive disease.
Notably, 1 pt who had only Dasatinib monotherapy without any form of chemotherapy nor allotransplant is still alive and in second chronic phase after more than two years of follow up.
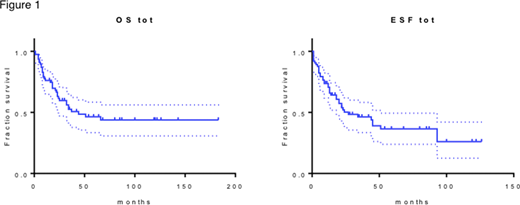
Median follow up for patients who received an active treatment was 23 months. Median OS was 44 months and median EFS was 29 months (figure 1). The only factor whose impact was significant for both OS and EFS in our cohort was transplant (p<0.0001, median OS not reached and median EFS was 93 months for transplanted patients).
At last follow up, 30 pts (47%) are still alive (29 in CR, 1 pt with active refractory disease), 30 pts (47%) died, 4 pts (6%) are lost to follow up. Causes of death were progression of disease for 18 pts, sepsis for 7 pts, GVHD for 3 pts, ischemic stroke for 1 pt, cardiovascular complications for 1 pt.
Conclusion :We showed here a multicentric experience about an unselected cohort of patients with MBC CML and Ph+ AML who received different treatments according to age, comorbidities, performance status, TKI availability. Survival of this cohort was mostly linked to the possibility to achieve CR and to perform an allotransplant. Already published experiences showed a generical dismal prognosis with median OS which doesn't exceed 1-2 years (16 months, Deau B, Leukemia Research 2011 ; 12 months, Palandri F, Haematologica 2008 ; 6.4 months, Fruehauf S, Cancer 2007) whereas in our real life cohort median OS was longer. For fit and potentially « transplantable » patients, combination with classic chemotherapy plus a TKI seems reasonable and associated with good outcomes in terms of OS and EFS.
Rousselot:Incyte:Consultancy, Research Funding;Pfizer:Consultancy, Research Funding;Bristol-Myers Squibb:Consultancy;Novartis:Consultancy;Takeda:Consultancy.Itzykson:Jazz Pharmaceuticals:Honoraria, Membership on an entity's Board of Directors or advisory committees;Daiichi Sankyo:Honoraria;BMS (Celgene):Honoraria;Sanofi:Honoraria;Astellas:Honoraria;Oncoethix (now Merck):Research Funding;Novartis:Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding;Janssen:Research Funding;Stemline:Membership on an entity's Board of Directors or advisory committees;Karyopharm:Membership on an entity's Board of Directors or advisory committees;Otsuka Pharma:Membership on an entity's Board of Directors or advisory committees;Amgen:Membership on an entity's Board of Directors or advisory committees;Abbvie:Honoraria.Cayuela:Incyte:Speakers Bureau;Novartis:Speakers Bureau.Rea:BMS:Membership on an entity's Board of Directors or advisory committees;Novartis:Honoraria, Membership on an entity's Board of Directors or advisory committees;Pfizer:Honoraria, Membership on an entity's Board of Directors or advisory committees;Incyte:Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal